vostok
Blooming
- User ID
- 156
Key points
• First time critical review of the heterologous process for recombinant THCA/CBDA production and critical review of bottlenecks and limitations for a bioengineered technical process
• Integrative approach of protein engineering, systems biotechnology, and biochemistry of yeast physiology and biosynthetic cannabinoid enzymes
• Comparison of NphB and CsPT aromatic prenyltransferases as rate-limiting catalytic steps towards cannabinoids in yeast as platform organisms
Introduction
Since the legalization of cannabis products for medicinal use, cannabinoids like tetrahydrocannabinol (THC) and cannabidiol (CBD) get attraction as for direct use or as potential drug candidates for various diseases. Besides isolation from plant material, the biotechnological production of cannabinoids like THC and CBD is an exciting alternative. Over the last years, the genetic blueprint of THC and CBD biosynthesis is understood, and genes have been functionally expressed in various microorganisms as documented in scientific papers. So far, no report has been communicated showing scaled up biosynthesis and how an industrial process must be designed to allow feasible economic production in a bioreactor. In this review, we take for the first time the endeavor to analyze basic biological parameters and to develop concepts and strategies for an engineered process to identify limitations, bottlenecks, and opportunities.
HERE:

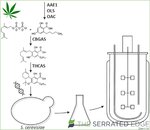
Tetrahydrocannabinolic acid (THCA) biosynthesic pathway in C. sativa L.. A total of six enzymes (AAE1, GPPS, OLS, OAC, CBGAS, THCAS)
form THCA from isopentenyl diphosphate (IPP) and dimethylallylphosphate (DMAPP) synthesized in the MEP pathway,
as well as hexanoic acid provided by the fatty acid biosynthesis
Conclusion
A systems biotechnology approach for high yield heterologous THCA biosynthesis is still in its infancy and must be characterized by low titers. Today, the question is not anymore what genes and biocatalysts must be used; the driving force in bioengineering 2.0 is to make kg and not mg to be competitive with plants. After careful analysis of metabolic bottlenecks in the heterologous THCA production, we can identify as critical the following:
- 1
Insufficient hexanoic acid formation in the fatty acid biosynthesis - 2
Low acetyl-CoA precursor delivery to the hexanoic acid biosynthesis and the mevalonate pathway - 3
Limiting catalytic activity of NphB or CsPT and THCAS - 4
Insufficient ATP and NADPH regeneration - 5
Ethanol production by Crabtree effect
A combination of the kinetics provided by Chen et al. (2012) and Förster et al. (2003) are used for the throughput calculation of glycolysis (Förster et al. 2003; Sheng and Feng 2015). The reactions of acetyl-CoA to isopentenyl pyrophosphate and dimethylallyl pyrophosphate were modeled with the data and kinetics of Smallbone et al. (2013) and the geranyl pyrophosphate synthase with the data of Ku et al. (2005).
THCA production by a genetically engineered S. cerevisiae strain was successfully modeled, and a bioprocess was in silico designed that allows prediction of industrial applications. Modeled pathways and obtained data showed clearly that the supply of GPP and olivetolic acid from the primary pathways is limiting the THCA biosynthesis in yeast. Especially low concentration of hexanoic acid is critical for high yield production of THCA. This can be solved but must be considered the top priority for any metabolic engineering of the yeast. We have not discussed other aspects of rational and smart metabolic engineering like transcription factors, import and export of substrate, and THCA as final products, but these must be validated as well in future modeling approaches. With all limitations, S cerevisiae is still the best platform organism we have to produce heterologously cannabinoids, but it is obvious that a perfect running yeast will not compete with C. sativa L. varieties known for THC concentrations of 20 % and more. Plant extraction will stay for a long time the first choice for THC delivery. But the cannabis biotechnology has its unique niche for the production of so-called minor or rare cannabinoids, which are present at very low concentrations of less than 0.5 % in dried flowers. Smart bioengineering will be an attractive alternative to the cannabis plant.
The Bottom Line:
In future the need for corporations to increase profitability it will be easier and very much cheaper to buy your THC/CBD from a lab in Europe derived from yeast than to use extracts from grown plants..
..as many of us have long suspected
Attachments
Last edited:






